Jurisdiction:
United States
Organ System:
Uterus
Funding Organization:
- National Cancer Institute, USA
Research Organizations:
- Baylor College of Medicine, USA
- New York University School of Medicine, USA
- Washington University in St. Louis, USA
- Pacific Northwest National Laboratory, USA
- Broad Institute of MIT and Harvard, USA
- Brigham Young University, USA
- University of Michigan, USA
- Icahn School of Medicine at Mount Sinai, USA
- National Cancer Institute, USA
Principal Investigators
:- David Fenyo
- Tao Liu
- Bing Zhang
- Li Ding
- Karin D. Rodland
Publication:
External Links:
The National Cancer Institute’s Clinical Proteomic Tumor Analysis Consortium (CPTAC) is a national effort to accelerate the understanding of the molecular basis of cancer through the application of large-scale proteome and genome analysis, or proteogenomics. Through a coordinated effort by CPTAC-affiliated Proteome Characterization Centers, Proteogenomic Translational Research Centers, and Proteogenomic Data Analysis Centers, CPTAC aims to comprehensively characterize human cancers.
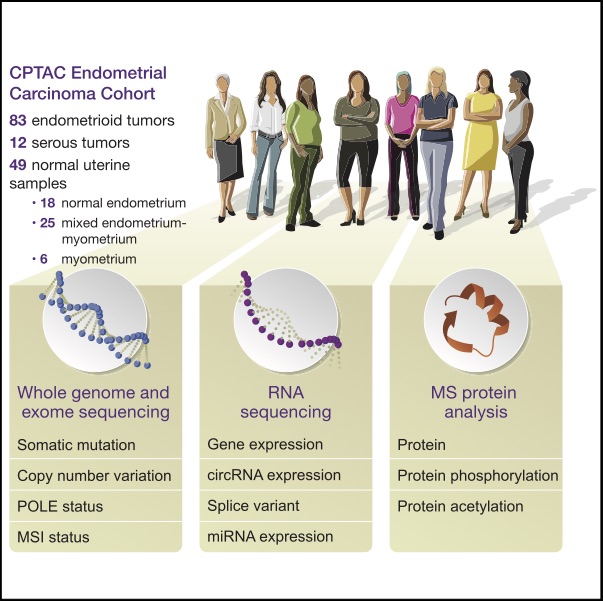
We undertook a comprehensive proteogenomic characterization of 95 prospectively collected endometrial carcinomas, comprising 83 endometrioid and 12 serous tumors. This analysis revealed possible new consequences of perturbations to the p53 and Wnt/b-catenin pathways, identified a potential role for circRNAs in the epithelial-mesenchymal transition, and provided new information about proteomic
markers of clinical and genomic tumor subgroups, including relationships to known druggable pathways. An extensive genome-wide acetylation survey yielded insights into regulatory mechanisms linking Wnt signaling and histone acetylation. We also characterized aspects of the tumor immune landscape, including immunogenic alterations, neoantigens, common cancer/testis antigens, and the immune
microenvironment, all of which can inform immunotherapy decisions. Collectively, our multi-omic analyses provide a valuable resource for researchers and clinicians, identify new molecular associations of potential mechanistic significance in the development of endometrial cancers, and suggest novel approaches for identifying potential therapeutic targets.