Jurisdiction:
China
Organ System:
Pancreas
Funding Organizations:
- National Key Research and Development Program of China
- National Natural Science Foundation of China
- Strategic Priority Research Program of the Chinese Academy of Sciences
- Shanghai Leading Talent Program of Eastern Talent Plan
- Sailing Project of Science and Technology Commission of Shanghai Municipality
- China Postdoctoral Science Foundation
- Shanghai Municipal Science and Technology Major Project
- Scientific Innovation Project of Shanghai Education Committee
- Baidu Foundation of China
- Clinical Research Plan Shanghai Hospital Development Center
- Science Foundation of Peking University Cancer Hospital
- CAS project for young scientists in basic research
- Xuhui District Intelligence Medical Hospital Cooperation Project
Research Organizations:
- Fudan University, China
- Peking University Cancer Hospital & Institute, China
- Chinese Academy of Sciences, China
Principal Investigators
:- Xianjun Yu
- Jianmin Wu
- Hu Zhou
- Gang Jin
- Daming Gao
Publication:
External Links:
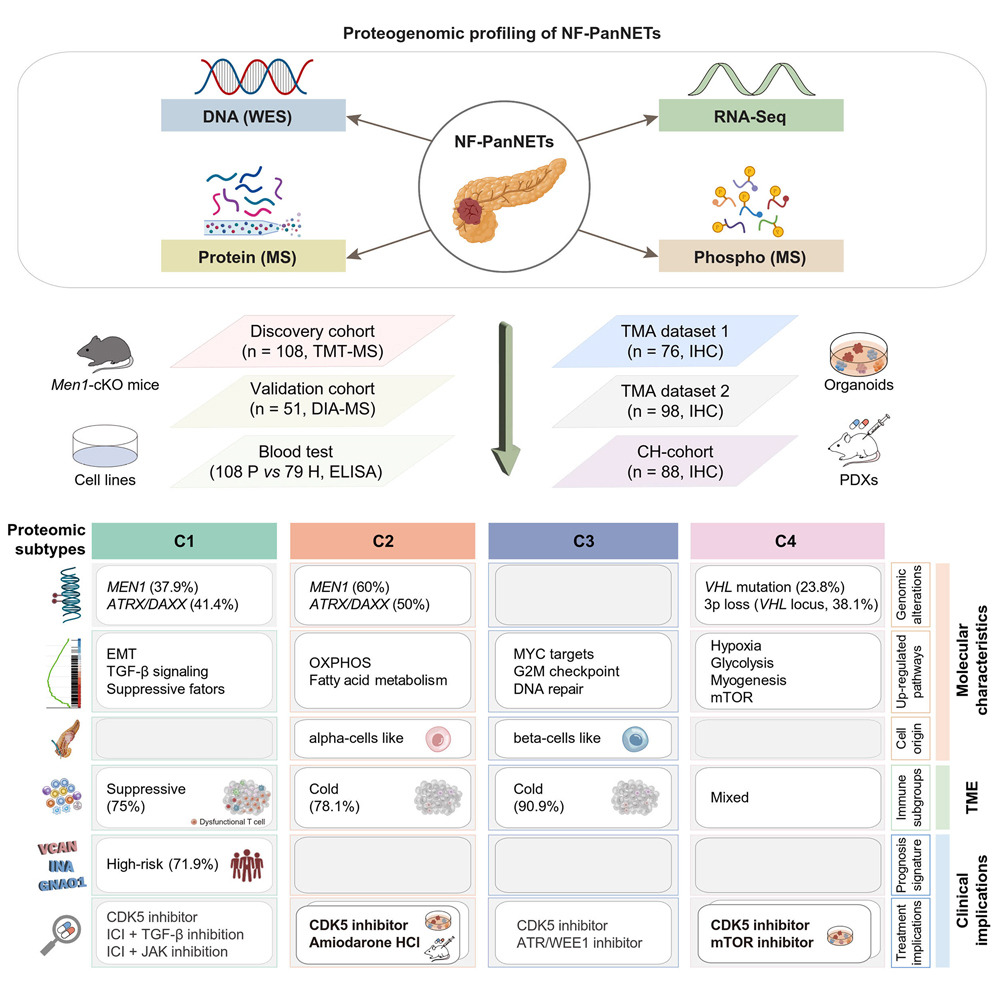
The majority of neuroendocrine neoplasms in pancreas are non-functional pancreatic neuroendocrine tumors (NF-PanNETs), which exhibit a high occurrence of distant metastases with limited therapeutic options. Here, we perform a comprehensive molecular characterization of 108 NF-PanNETs through integrative analysis of genomic, transcriptomic, proteomic, and phosphoproteomic profiles. Proteogenomic analysis provides functional insights into the genomic driver alterations of NF-PanNETs, revealing a potential mediator of MEN1 alterations using Men1-conditional knockout mice. Machine-learning-based modeling uncovers a three-protein signature as an independent prognostic factor, which is validated by an independent external cohort. Proteomic and phosphoproteomic-based stratification identifies four subtypes with distinct molecular characteristics, immune microenvironments, and clinicopathological features. Drug screening using patient-derived tumor organoids identifies cyclin-dependent kinase (CDK) 5 and Calcium Voltage-Gated Channel Subunit Alpha1 D (CACNA1D) as ubiquitous and subtype-specific targets, respectively, with in vivo validation using xenograft models. Together, our proteogenomic analyses illustrate a comprehensive molecular landscape of NF-PanNETs, revealing biological insights and therapeutic vulnerabilities.