Jurisdiction:
United States
Organ System:
Ovary
Funding Organization:
- National Cancer Institute, USA
Research Organizations:
- Pacific Northwest National Laboratory, USA
- Oregon Health & Science University, USA
- Johns Hopkins University, USA
- Brigham Young University, USA
- Baylor College of Medicine, USA
- Washington University in St. Louis, USA
- National Cancer Institute, USA
Principal Investigators
:- Karin D. Rodland
- Tao Liu
- Hui Zhang
- Daniel W. Chan
Publication:
External Links:
The National Cancer Institute’s Clinical Proteomic Tumor Analysis Consortium (CPTAC) is a national effort to accelerate the understanding of the molecular basis of cancer through the application of large-scale proteome and genome analysis, or proteogenomics. Through a coordinated effort by CPTAC-affiliated Proteome Characterization Centers, Proteogenomic Translational Research Centers, and Proteogenomic Data Analysis Centers, CPTAC aims to comprehensively characterize human cancers.
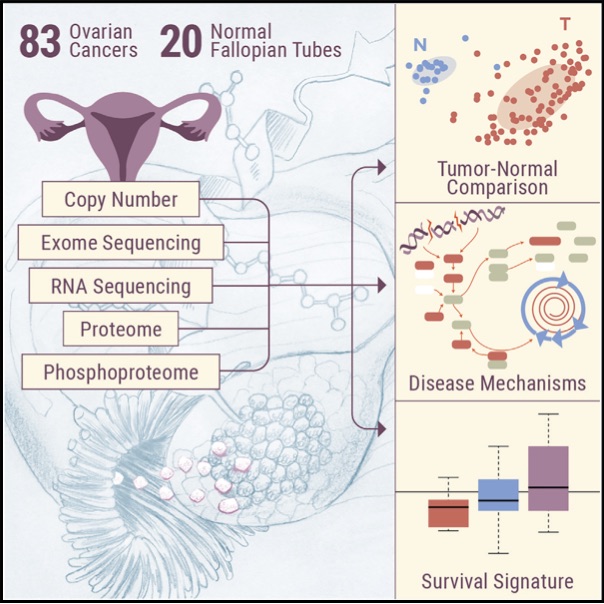
In the absence of a dominant driving mutation other than uniformly present TP53 mutations, deeper understanding of the biology driving ovarian high-grade serous cancer (HGSC) requires analysis at a functional level, including post-translational modifications. Comprehensive proteogenomic and phosphoproteomic characterization of 83 prospectively collected ovarian HGSC and appropriate normal precursor tissue samples (fallopian tube) under strict control of ischemia time reveals pathways that significantly differentiate between HGSC and relevant normal tissues in the context of homologous repair deficiency (HRD) status. In addition to confirming key features of HGSC from previous studies, including a potential survival-associated signature and histone acetylation as a marker of HRD, deep phosphoproteomics provides insights regarding the potential role of proliferation-induced replication stress in promoting the characteristic chromosomal instability of HGSC and suggests potential therapeutic targets for use in precision medicine trials.